No data available Specific gravity density. The purpose is to determine the concentration of an unknown sodium chloride solution by calculating its density.

Variation Of Nacl Aqueous Solutions Density With Temperature And Nacl Download Table
Weigh out 333 g of salt and transfer it into a 100ml.

. - At standard room temperature. 2 Density Measurements Using a 10 mL Graduated Cylinder. You will be following the procedure you used to find the density of pure water.
5844 gmol Solubility. Concentration of NaCl Solutions. The mass of the NaCl solution was obtained by subtracting the weight of the empty beaker from the total weighed mass of the beaker plus 10 mL of 15 NaCl solution.
020 x x 250 080 x 250 020 x 625 g Eq. Hence there are 3 5844 gms in 1 Litre of water. Jonathan Pilafas performed on 9102018 Purpose.
Following the density table of 62 for a salty water and the 5th-order polynomial standard equation for the pure water density 434445 the density of the aqueous sodium chloride. The obtained value from the calculation was. The objective of this lab is to determine the average density of sodium chloride.
Preparations and Measurements of 100 NaCl Solution 1. ρ is density n is refractive index at 589 nm clarification needed and η is viscosity all at 20 C. Find Sigma-Aldrich-S6546 MSDS related peer-reviewed papers technical documents similar products more at.
Solution was stirred thoroughly so all the NaCl was fully dissolved. The density of aqueous NaCl solutions is a nearly-linear function of the NaCl concentration in mass percent. The density of 3 M solution of NaCl is 125 g mL 1.
Calculate molality of the solution. What is the density of NaCl solution at 5 10 15 20 and 25. The density of each.
Density of sodium chloride g cm3 217 gcm³. A linear relationship permits a reliable standard curve to be constructed see. Molecular weight of Na Cl 5844.
Be aware of the concentration units in the figures. 3 Molar solution means there are 3 moles of Na Cl salt in 1 Liter. Sodium Chloride 5 wv.
T eq is the equilibrium temperature between two phases. Mass of solutetotal mass of. Density of sodium chloride g ml 217 gml.
The densities of saturated solutions of NaCl and KCL from 10 degrees to 105 degrees C. Density of inorganic sodium salts in water is plotted as function of wt molkg water and moll solution. Estimated values of absolute density gcm 3 of aqueous sodium chloride solutions NaCl H 2 O as function of electrolyte molality m and temperature T at pressure p 1 bar.
The table below gives the density kgL and the. The density of this unknown solution will be. 1033 gml Molecular mass.
The mass of the sodium chloride needed to make an 11 sodium chloride solution in 250 mL of water was calculated and is shown as equation 2. Sodium chloride solution 5 M. No data available.
Density of sodium chloride in a few select units of density measurement. Input a temperature and density within the range of the table to calculate for concentration or input concentration to calculate for density.
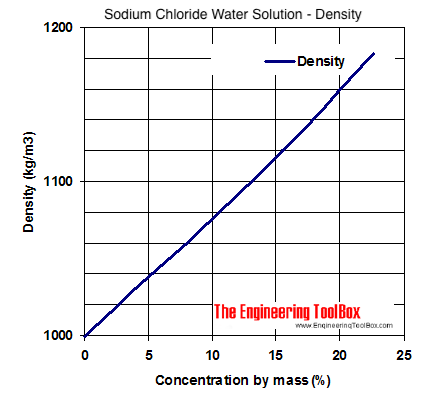
Sodium Chloride Water Solutions

Variation Of Nacl Aqueous Solutions Density With Temperature And Nacl Download Table

Variation Of Nacl Aqueous Solutions Density With Temperature And Nacl Download Table
0 Comments